Oven cleaners have become a staple in most kitchens, helping homeowners get rid of stubborn grease and burnt food residues that regular soapy water can’t tackle. But have you ever wondered about the science behind these cleaners, particularly their pH levels?
The common pH of oven cleaners is typically alkaline, often around 11-13. An alkaline pH helps break down greasy stains more effectively.
In this guide, look more closely into the pH world of oven cleaners, why it matters, and how it impacts your kitchen appliances and cooking.
What Does pH mean?
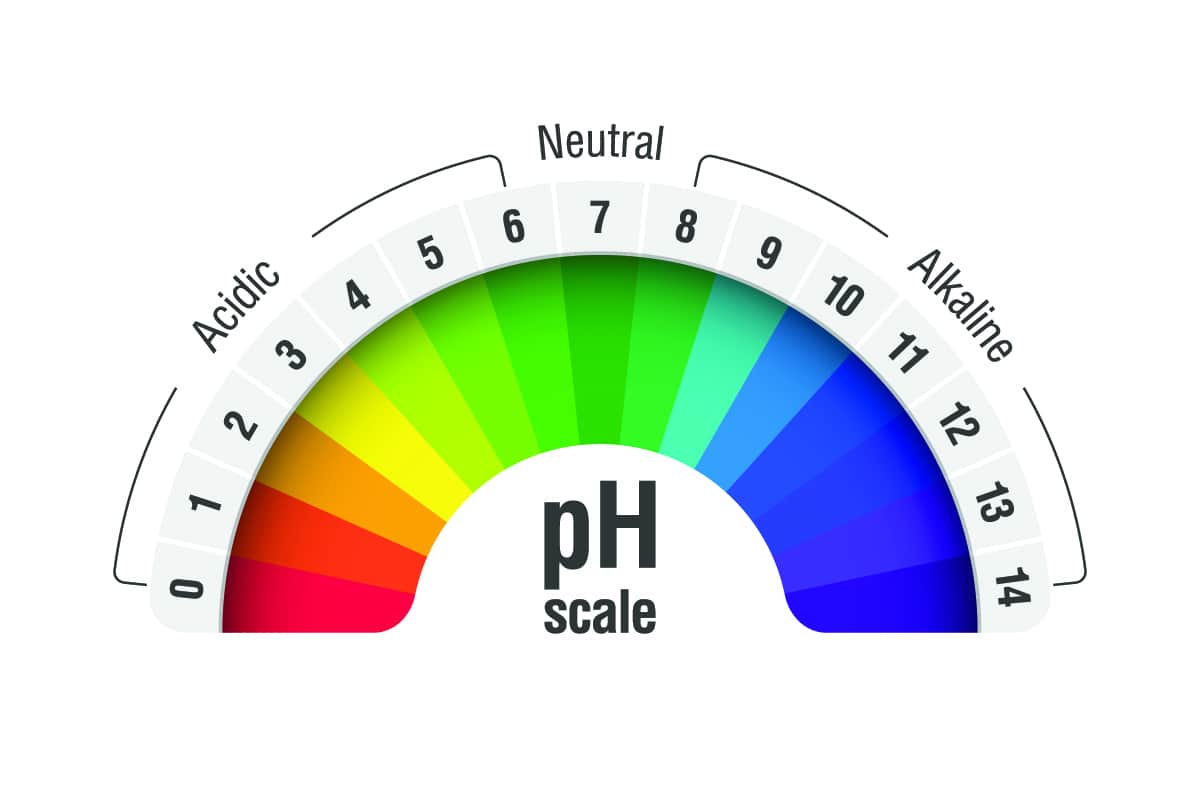
pH stands for “potential of hydrogen.” Think of it as a scale that tells us how acidic or alkaline a substance is. This scale ranges from 0 to 14.
The middle of the scale, 7, is neutral—like water. Numbers below 7 are acidic (lemon juice, coffee), and numbers above 7 are alkaline (baking soda, soap).
Different pH levels can be more or less effective at various tasks. For instance, acidic solutions are great for removing rust, while alkaline solutions, like oven cleaners, tackle grease like a pro.
Everyday items, from beverages to cleaning products, have their spot on the pH scale. Being aware of their pH can help in using them effectively. For example, knowing that vinegar is acidic can help when you’re trying to conquer calcium deposits.
pH of Common Household Substances
Ever spilled coffee on your shirt and wondered why it’s so stubborn? Or why vinegar makes an excellent cleaner for certain messes? It’s all in the pH!
Edibles
- Citrus Fruits: These zesty delights, like lemons and oranges, are acidic. They usually sit around a pH of 2-3. That’s why lemon juice can be used as a natural cleaner.
- Baking Soda: Ah, the mighty rising agent for your cookies. Baking soda is alkaline, generally clocking in at a pH of 9.
- Milk: This one’s a tad acidic, usually settling around a pH of 6.5. Hence, the sour taste when it’s past its prime.
- Black Coffee: Before you add cream or sugar, coffee’s pH is typically around 5, making it slightly acidic. Maybe that’s why it “wakes” you up!
Cleaning Agents
- Vinegar: White vinegar, the cleaning hero of many households, is acidic, with a pH value of about 2.5. Great for shining up those faucets!
- Bleach: This heavyweight cleaner is on the alkaline side, usually with a pH of around 12. Definitely not for cocktail hour.
- Dish Soap: Most dish soaps lean alkaline, with pH levels around 7-8. Perfect for those greasy pans.
Hygiene Products
- Shampoos: Depending on the type, shampoos can vary, but they’re often slightly acidic, around a pH of 5-6, to balance the scalp’s natural oils.
- Hand Soap: Liquid hand soaps are generally neutral to slightly alkaline, with pH levels ranging from 7 to 9.
Determining the pH of Oven Cleaner

Determining the pH level of oven cleaners isn’t some high-flying science; it’s a piece of cake!
Why Bother Checking?
Firstly, knowing the pH helps you use the product more effectively. An oven cleaner’s pH tells you how good it is at tackling those stubborn stains.
Methods to Test pH
- pH Test Strips: These are like magical litmus papers. Dip, compare, and voilà! You know the pH.
- Digital pH Meters: A bit techy but super accurate. Just a dab of your oven cleaner, and it gives you the number.
- Reading Labels: Some brands save you the fuss and mention the pH right there.
Why Does Oven Cleaner Need to Be Alkaline?
It’s about the cleaning power these products pack, thanks to their alkaline nature.
- Safety First: Alkaline solutions can be strong. So, while they’re fabulous for cleaning, it’s a good idea to wear gloves. Keep those hands as soft as your freshly cleaned oven!
- Grease and Grime Be Gone: Greasy stains are like the uninvited guests of your oven. Alkaline substances break down fats and oils. So, an alkaline oven cleaner acts like the bouncer, showing those greasy gatecrashers the door.
- Natural vs. Chemical: While many store-bought oven cleaners are chemically alkaline, there are natural alkaline substances, too. For instance, baking soda, a natural alkaline, can also help in cleaning ovens, albeit with a bit more elbow grease.
- Alkaline and Environment: Alkaline substances, when used right, tend to be more eco-friendly. They break down easily and don’t linger around, unlike some acidic cleaners.
Effects of Oven Cleaner pH on Appliances and Cooking

Pull up a chair and grab your detective hat. Let’s learn about how the pH of your oven cleaner impacts your prized kitchen gadgets.
Oven Surfaces
- Non-stick Coating: Ever noticed that sleek, shiny non-stick surface inside some ovens? Alkaline cleaners can be a bit too aggressive for these, potentially causing wear over time.
- Stainless Steel: Good news for stainless steel oven owners! Alkaline cleaners and stainless steel go together like peanut butter and jelly. They clean without causing harm.
- Glass Doors: Those oven windows can take the alkaline hit pretty well. But too much elbow grease might leave scratches, so gentle scrubbing is your BFF.
Oven Performance and Longevity
- Heating Element: Residue from oven cleaners, especially if it’s alkaline, shouldn’t hang around the heating elements. It could cause a little wear and tear over time.
- Efficiency: A clean oven heats more efficiently. So, while you’re making sure your oven’s insides shine, you’re also ensuring your food cooks evenly.
- Odors: Ever noticed a funky smell when you turn on your oven? Cleaning residues could be the culprits. Post-clean, always do a dry run at a high temperature to let any leftover cleaner evaporate.
Cookware
- Aluminum Pans: Alas, our alkaline cleaners are not the best buddies with aluminum pans. They can cause discoloration. After a cleaning spree, maybe give these pans a rinse before the next use.
- Cast Iron Drama: Cast iron pans have a seasoning (a layer of baked-on oil). Alkaline cleaners can strip this away, so if your oven’s had a deep clean, re-season your pan before its next outing.
Food Safety and Taste
- Residue: Leftover oven cleaner residue could impact food taste. Always give the oven a good rinse and heat cycle post-clean. No one likes their pie with a hint of “eau de cleaner”.
- Safety: Modern oven cleaners are formulated with safety in mind. But, it’s always best to remove all traces before firing up that oven. That roast chicken deserves a clean, chemical-free space.
FAQs
1. Is it safe to use an oven immediately after cleaning?
After all that scrubbing, it’s best to give it a little time to relax. If you’ve used a chemical cleaner, heat the oven empty for about 15 minutes to get rid of any leftover cleaner vibes. For natural cleaners, just give it a quick rinse and you’re good to go!
2. What can I use as a natural oven cleaner?
Mix equal parts baking soda and water to form a paste. Slap that concoction on your oven’s grimy spots and let it sit overnight. The next day, spritz some vinegar on it, scrub it away, and bask in the glory of a naturally clean oven. Bonus tip: Lemon juice works wonders too!
3. How do I know if my oven cleaner is too acidic or too alkaline?
Most oven cleaners lean towards the alkaline side because that’s where the grease-fighting magic happens. If you want to get all Sherlock Holmes about it, grab some pH test strips from a store. If it’s showing numbers below 7, it’s acidic. Numbers above 7? Alkaline territory. But generally, just follow the label instructions, and your oven will thank you with some spotless shine!